 The genome sequence of the gray short-tailed opossum, Monodelphis domestica, was published in today’s issue of Nature (Mikkelsen et al. 2007). It is interesting for many reasons, including its status as the first marsupial genome to be sequenced, its relatively large genome size, and low chromosome number (2n = 18). It is also interesting because it contains a similar number of genes (18,000 – 20,000) to humans, the vast majority of which exhibit close associations with the genes of placental mammals. Also, in keeping with the hypothesis that transposable elements are the dominant type of DNA in most eukaryotic genomes, the comparatively large opossum genome is comprised of 52% transposable elements, the most for any amniote sequenced so far.
The genome sequence of the gray short-tailed opossum, Monodelphis domestica, was published in today’s issue of Nature (Mikkelsen et al. 2007). It is interesting for many reasons, including its status as the first marsupial genome to be sequenced, its relatively large genome size, and low chromosome number (2n = 18). It is also interesting because it contains a similar number of genes (18,000 – 20,000) to humans, the vast majority of which exhibit close associations with the genes of placental mammals. Also, in keeping with the hypothesis that transposable elements are the dominant type of DNA in most eukaryotic genomes, the comparatively large opossum genome is comprised of 52% transposable elements, the most for any amniote sequenced so far.
One of the most intriguing discoveries about the opossum genome is that changes to protein-coding genes seem not to have been the driving force behind mammalian diversification. Instead, non-coding elements with regulatory functions — mostly derived from formerly parasitic transposable elements — appear to underly much of the difference.
Now, I would prefer to just talk about the science here, noting that this is yet another great example of the complex nature of genome evolution, the key role played by “non-standard” genetic processes (Gregory 2005), and the ever-increasing relevance of non-coding DNA in genomics. But, inevitably, I must comment on how this discovery has been reported. Here is what ScienceDaily (which I otherwise like a great deal) said about it:
Opossum Genome Shows ‘Junk’ DNA Source Of Genetic Innovation
(…)
The research, released Wednesday (May 9) also illustrated a mechanism for those regulatory changes. It showed that an important source of genetic innovation comes from bits of DNA, called transposons, that make up roughly half of our genome and that were previously thought to be genetic “junk.”
The research shows that this so-called junk DNA is anything but, and that it instead can help drive evolution by moving between chromosomes, turning genes on and off in new ways.
(…)
It had been initially thought that most of a creature’s DNA was made up of protein-coding genes and that a relatively small part of the DNA was made up of regulatory portions that tell the rest when to turn on and off.
As studies of mammalian genomes advanced, however, it became apparent that that view was incorrect. The regulatory part of the genome was two to three times larger than the portion that actually held the instructions for individual proteins.
I will just reiterate two brief points, as I have already dealt with some of these topics in earlier posts (and will undoubtedly have to do so again in the future). One, very few people have actually argued that all non-coding DNA is 100% functionlesss “junk”, and no one is surprised anymore when a regulatory or other function is observed for some non-coding DNA sequences. Moreover, transposable elements are more commonly labeled as “selfish DNA”, and it has been noted in countless articles that they can and do take on functions at the organism level even if they begin as parasites at the genome level. Two, yet again we are talking about a small portion of the genome such that this should not be considered a demonstration that all non-coding DNA is functional. In particular, the authors identified about 104 million base pairs of DNA that is conserved (i.e., shared and mostly invariant) among mammals, about 29% of which overlapped with protein-coding genes. In other words, about 74 million base pairs of non-coding DNA, much of it derived from former transposable elements, is found to be conserved among mammals and shows signs of being functional in regulation. The genome size of the opossum is probably around 3,500 million bases, which means that this functional non-coding DNA makes up 2% of the genome.
A note to science writers. There is nothing surprising about some sequences of non-coding DNA having an important function. The notion that all non-coding DNA has long been assumed to be completely functionless junk is a straw man. And to avoid misleading readers, you really need to specify that most examples of non-coding DNA with a function represent a very small portion of the total genome.
___________
References
Gregory, T.R. 2005. Macroevolution and the genome. In The Evolution of the Genome (ed. T.R. Gregory), pp. 679-729. Elsevier, San Diego.
Mikkelsen, T.S., M.J. Wakefield, B. Aken, C.T. Amemiya, J.L. Chang, S. Duke, M. Garber, A.J. Gentles, L. Goodstadt, A. Heger, J. Jurka, M. Kamal, E. Mauceli, S.M.J. Searle, T. Sharpe, M.L. Baker, M.A. Batzer, P.V. Benos, K. Belov, M. Clamp, A. Cook, J. Cuff, R. Das, L. Davidow, J.E. Deakin, M.J. Fazzari, J.L. Glass, M. Grabherr, J.M. Greally, W. Gu, T.A. Hore, G.A. Huttley, M. Kleber, R.L. Jirtle, E. Koina, J.T. Lee, S. Mahony, M.A. Marra, R.D. Miller, R.D. Nicholls, M. Oda, A.T. Papenfuss, Z.E. Parra, D.D. Pollock, D.A. Ray, J.E. Schein, T.P. Speed, K. Thompson, J.L. VandeBerg, C.M. Wade, J.A. Walker, P.D. Waters, C. Webber, J.R. Weidman, X. Xie, M.C. Zody, J.A.M. Graves, C.P. Ponting, M. Breen, P.B. Samollow, E.S. Lander, and K. Lindblad-Toh. 2007. Genome of the marsupial Monodelphis domestica reveals innovation in non-coding sequences. Nature 447: 167-177.


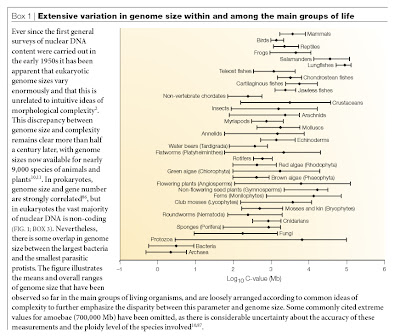





Ryan Gregory has serious doubts about the usefulness of the term as he explains in his excellent article A word about “junk DNA”.
Just to clarify, I think the term could be useful — indeed, it was useful when Ohno coined it. The problem is that it is seldom used in an appropriate way. If the meaning were specified explicitly to be “regions strongly suspected of being non-functional with evidence to back it up” (which, incidentally, is not the original definition according to Ohno (1972) or Comings (1972)), and if people used it only in this way, then I would not have a problem with this. But given the difficulty that people seem to have in accepting that some DNA may truly not have a function at the organism level, I don’t know if we could ever get it to be used with such precision.
…a new term, Junctional DNA, to describe DNA that probably has a function but that function isn’t known… think we don’t need to go there. It’s sufficient to remind people that lots of DNA outside of genes has a function and these functions have been known for decades.
That neologism was suggested in response to Minkel’s appeal for a term that would “make the distinction between functional and nonfunctional noncoding DNA clear to a popular audience”. My main suggestion was to call DNA by what it is known to be, if at all possible, by function (“regulatory DNA”, “structural DNA”) or by type (“pseudogene”, “transposable element”, “intron”). Your definition of “junk DNA” is also more precise than most usages, meaning that you specify that the term only be applied to sequences for which there is evidence (not just assumption) of non-function. That leaves us with something in between for journalists to talk about with a catchy buzzword. “Junctional DNA” lets them specify that we’re not talking about “junk DNA” or “functional DNA” — i.e., there is some evidence for function (e.g., being conserved) but no evidence of what that function is. The main utility would be to stop the very frustrating leap that gets made from “this 1% of the genome may have a function, so the whole thing must have this function” kind of reporting. Now they could say “another 1% has moved into the category of ‘junctional DNA'”. I think that would be considerably less misleading than current wording.
Note that I’m avoiding the term “noncoding” DNA here. This is because to me the term “coding DNA” only refers to the coding region of a gene that encodes a protein … there are many genes for RNAs that are not properly called coding regions so they would fall into the noncoding DNA category … introns in eukaryotic genomes would be “noncoding DNA” as far as I’m concerned. I think that Ryan Gregory and others use the term “noncoding DNA” to refer to all DNA that’s not part of a gene instead of all DNA that’s not part of the coding region of a protein encoding gene. I’m not certain of this.
By definition, non-coding DNA is, and always has been, everything other than exons. The reason this is relevant is that early work in genome biology assumed that there should be a 1 to 1 correspondence between DNA content and protein-coding gene number. This is work that occurred for at least two decades before the discovery of introns, pseudogenes, and other non-coding DNA. Now we have more descriptive names for the categories of DNA that are not the genes, all the genes, and nothing but the genes. I actually don’t know of anyone else who would have a problem calling introns, pseudogenes, and regulatory regions “non-coding DNA”. Certainly, Ohno, Crick, and many others have historically put introns in the same non-protein-coding grouping as pseudogenes. It’s just a category — you also have more specific subcategories to apply to each of the types of non-coding DNA. Perhaps your objection relates to an undue emphasis on the distinction between exons and everything else — well, that’s the history of the past half century of this field, so it should be no surprise that the terminology reflects this.
Read Gregory’s article for the short concise version of this dispute. What it means is that junk DNA threatens the worldviews of both Dembski and Dawkins!
Not quite. What you’re leaving out of this is the possibility of multiple levels of selection. In the original edition of The Selfish Gene (1976, p.76), Dawkins argued that “the simplest way to explain the surplus DNA is to suppose that it is a parasite, or at best a harmless but useless passenger, hitching a ride in the survival machines created by the other DNA”. Cavalier-Smith (1977) drew a similar conclusion (before he had read Dawkins), and Doolittle and Sapienza (1980) and Orgel and Crick (1980) [yes, that Crick] independently developed the concept of “selfish DNA” a few years later. This is an explicitly multi-level selection approach because it specifies that non-coding DNA can be present due to selection within the genome rather than exclusively on the organism (or gene, in Dawkins’s case) (see, e.g., Gregory 2004, 2005). (Incidentally, this idea of parasitic DNA dates back at least to 1945, when Gunnar Östergren characterized B chromosomes in this fashion). Of course, they tended to do what Ohno did and applied this one idea to all non-coding DNA, which is too ambitious. The modern view is more pluralistic (see, e.g., Pagel and Johnstone 1992 vs. Gregory 2003). Some non-coding DNA is just accumulated “junk” (in the definition of evidence-supported non-function that you espouse). Some (perhaps most) is “selfish” or “parasitic” and persists because there is selection within the genome as well as on organisms (in fact, an argument could be, and has been, made that “selfish DNA” would be a much more accurate term than “junk DNA” for most non-coding DNA). Some non-coding DNA is clearly functional at the organism level, including regulatory regions and chromosome structure components. Some of these latter functional non-coding DNA sequences are derived from elements that originally were of one of the first two types, most notably transposable elements that take on a regulatory function through co-option (or, in another manner of thinking, that undergo a shift in level of selection).
Junk DNA is not noncoding DNA and anyone who claims otherwise just doesn’t know what they’re talking about.
I’m afraid I don’t follow what you mean here. By your definition, “junk DNA” is any non-functional sequence of DNA, including pseudogenes (i.e., the original meaning). Those sequences do not encode proteins. Hence, your version of junk DNA is non-coding. I think this reflects the confusion that is imposed by the term “junk DNA”, which is why I generally think it is more obfuscating than enlightening.
________
References
Cavalier-Smith, T. 1977. Visualising jumping genes. Nature 270: 10-12.
Comings, D.E. 1972. The structure and function of chromatin. Advances in Human Genetics 3: 237-431.
Dawkins, R. 1976. The Selfish Gene. Oxford University Press, Oxford.
Doolittle, W.F. and C. Sapienza. 1980. Selfish genes, the phenotype paradigm and genome evolution. Nature 284: 601-603.
Gregory, T.R. 2003. Variation across amphibian species in the size of the nuclear genome supports a pluralistic, hierarchical approach to the C-value enigma. Biological Journal of the Linnean Society 79: 329-339.
Gregory, T.R. 2004. Macroevolution, hierarchy theory, and the C-value enigma. Paleobiology 30: 179-202.
Gregory, T.R. 2005. Macroevolution and the genome. In The Evolution of the Genome (ed. T.R. Gregory), pp. 679-729. Elsevier, San Diego.
Ohno, S. 1972. So much “junk” DNA in our genome. In Evolution of Genetic Systems (ed. H.H. Smith), pp. 366-370. Gordon and Breach, New York.
Orgel, L.E. and F.H.C. Crick. 1980. Selfish DNA: the ultimate parasite. Nature 284: 604-607.
Östergren, G. 1945. Parasitic nature of extra fragment chromosomes. Botaniska Notiser 2: 157-163.
Pagel, M. and R.A. Johnstone. 1992. Variation across species in the size of the nuclear genome supports the junk-DNA explanantion for the C-value paradox. Proceedings of the Royal Society of London, Series B: Biological Sciences 249: 119-124.